Rapid systematic reviews delivering the Body of Evidence (BOE)
to accelerate research and planning in the life sciences
A global specialist group with 20+ years building clinical evidence
case resources for marketing and economic strategies
ACCELERATE
EVALUATE
DE-RISK
CONVENIENCE
WHAT WE DO
GIVING YOUR PRODUCT THE
STRATEGIC FEED ACCELERATOR ADVANTAGE
The Strategic Feed™ Accelerator team delivers quickly, accurately and in ready to use formats, the evidence decision makers need to inform commercial decisions, generate new marketing materials, feed into market access & regulatory submissions and win approvals for new and existing products. Our menu of products range from performing a systematic search to providing the strategy, approach, case studies, analyses and presentation report in a format fit for purpose.
We provide client management with the necessary information to ascertain the ROI and commercial viability of a business issue, by performing project feasibilities that assess the following:
WHY CLIENTS ENGAGE US

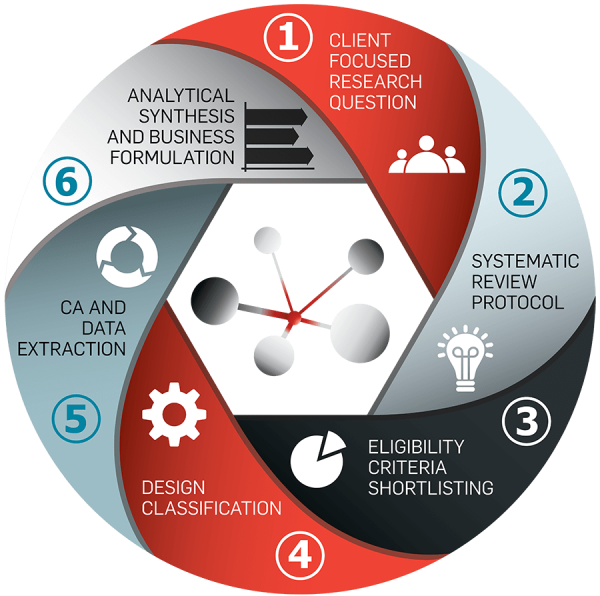
OUR PROCESS
WHO WE ARE
An international team of evidentiary, marketing and commercial experts; managing clinical evidence across three continents with over 20 years specialist expertise in building successful evidence-case resources for marketing and economic strategies.
The Strategic Feed team have considerable experience developing integrated strategies across HEOR, RWE, Phase IIIb/IV, and all other data supported by microanalysis such as HTAs and systematic reviews throughout the product lifecycle, from concept-to-post-marketing activities. This tailors information to specific issues of therapeutic and/or marketing importance.
WHAT CAN WE EVALUATE FOR YOU?
Strategic Feed is a rapid systematic review protocol for feasibility assessments on areas of commercial enquiry such as expanded indications, licensing reviews, new data to motivate stakeholders (Drs, Reps), value assessments, bibliographic submissions, government initiatives. We can perform a feasibility study to scope out the project and to deliver the Body of Evidence (BOE) towards your research and planning.